You’ve specified beautiful, golden brass for your CNC machined components, appreciating its strength, machinability, and classic appearance. Yet, not long after production, you notice the brilliant shine fading, replaced by a dull, brownish, or even blackish discoloration. This tarnishing effect can make precision-engineered parts look aged and low-quality, undermining the value of your product.
This unexpected discoloration leads to aesthetic rejection, customer complaints, and potentially costly rework or refinishing processes. When a part designed for its visual appeal and durability starts to degrade, it creates doubt about the quality of the CNC machining and the material itself. You are left struggling with how to maintain that “fresh off the machine” look and protect your investment.
The solution lies in a clear understanding of the electrochemical reactions that cause brass to tarnish and in the proactive implementation of preventative measures. Tarnishing is a natural process, but it is controllable. Through careful material selection, proper handling during the CNC milling process, and the application of appropriate post-machining finishes, you can effectively preserve the luster and integrity of your CNC brass parts for the long term.

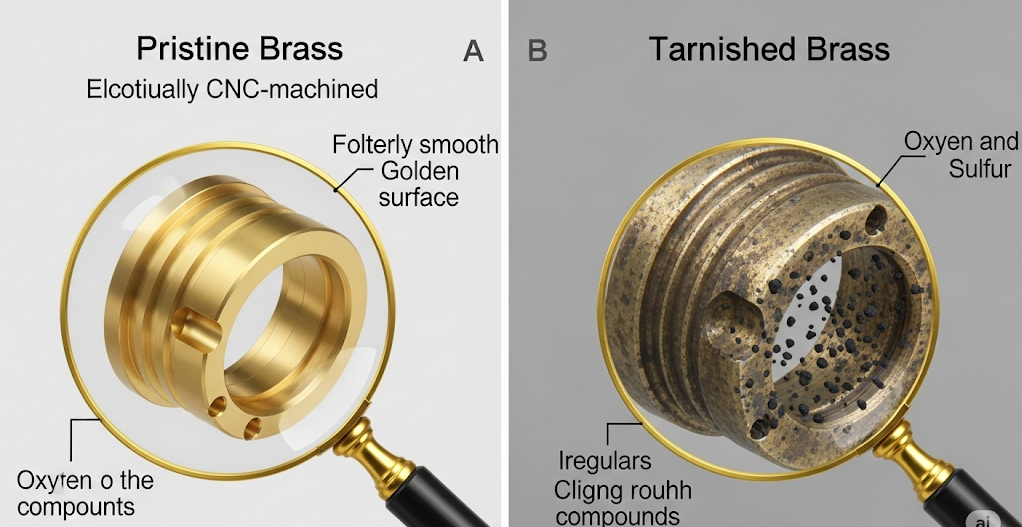
CNC machined brass parts turn black or tarnish due to an oxidative process. The copper and zinc in the brass alloy react with oxygen, moisture, and sulfur compounds in the atmosphere. This reaction forms a thin surface layer of metal oxides, sulfides, and carbonates, known as tarnish. Initially appearing as a dulling of the surface, this layer darkens over time from brown to dark gray or black as it thickens.
Now that we’ve pinpointed the fundamental cause of brass discoloration, we can dissect the various factors that influence its speed and severity. This article will therefore explore the specific chemistry of brass oxidation, clarifying why this popular alloy is susceptible to tarnishing. Subsequently, we will investigate how variables within the CNC machining environment itself can accelerate this process. Ultimately, this guide will provide a robust framework of preventative strategies and finishing options to keep your brass components looking their best.
The Inherent Chemistry of Brass and Tarnishing
Brass is an alloy, not a pure element. It is primarily composed of copper and zinc, and it’s this combination that gives brass its unique properties—its distinctive color, excellent machinability, and good corrosion resistance. However, it is also this combination, particularly the presence of copper, that makes it susceptible to tarnishing. The process is a complex electrochemical reaction with the surrounding environment.
The main driver is oxidation. Both copper and zinc are reactive metals that will combine with oxygen in the air. The copper component reacts to form copper oxides (first the reddish Cu₂O, then the black CuO), which is the primary cause of the darkening. Simultaneously, the zinc component also oxidizes to form zinc oxide (ZnO), typically a white or yellowish powder. The interplay between these reactions defines how the brass tarnishes.
Furthermore, the process is heavily accelerated by airborne pollutants, especially sulfur compounds like hydrogen sulfide (H₂S). These compounds are present in trace amounts everywhere, but are more concentrated in industrial areas. They react readily with the copper in the brass to form copper sulfide (Cu₂S), which is distinctly black and contributes significantly to a heavy tarnish. Moisture in the air acts as a catalyst, providing an electrolyte that facilitates all these chemical reactions. Therefore, a freshly cut part from a CNC machining center, with its highly activated surface, is in a prime state to begin tarnishing, especially in a humid environment.
How the CNC Machining Process Impacts Brass Tarnishing
While tarnishing is a natural tendency, the CNC machining process itself can create conditions that hasten its onset. A high-quality CNC milling operation, like those at ly-machining, is aware of these factors and takes steps to mitigate them. Understanding these influences is key to producing stable, tarnish-resistant parts.
1. Thermal Effects from Cutting
The friction generated at the point where a cutting tool meets the brass workpiece is intense. During CNC milling, this creates a localized spike in temperature on the newly machined surface. Although brass has good thermal conductivity and dissipates this heat well, this momentary thermal energy increases the surface’s chemical reactivity. A hotter surface reacts with oxygen and other atmospheric elements at a much faster rate, meaning the tarnishing process can be initiated fractions of a second after the material is cut.
2. Surface Finish and Area
Every CNC machining operation creates a new surface with a specific texture, or surface roughness (Ra). This texture consists of microscopic peaks and valleys left by the cutting tool. This intricate topography vastly increases the effective surface area of the part compared to a perfectly smooth plane. This larger surface area provides more material to be exposed to oxygen and moisture. These microscopic valleys can also trap contaminants and humidity, creating localized cells where corrosion and tarnishing can begin and thrive. A part with a rougher finish will generally tarnish more quickly than one with a fine, smooth finish.
3. The Critical Role of Cutting Fluids
Cutting fluids are indispensable for cooling, lubrication, and chip evacuation during the CNC machining of brass. However, their chemical composition can be a double-edged sword. If the coolant is not specifically formulated for “yellow metals” (copper, brass, bronze), it can be detrimental. Certain additives, especially those containing active sulfur, can cause immediate, aggressive staining and blackening. Moreover, if the coolant is not properly maintained, it can become a vector for contamination. Tramp oils, environmental pollutants, and bacterial growth can introduce sulfurous compounds into the fluid, which are then deposited onto the finished part, setting the stage for rapid tarnishing. This is a critical process control point for any quality-focused operation.
Understanding the Spectrum of Discoloration
The color change on brass isn’t always a simple path to black. The term “patina” encompasses the various stable surface layers that can form. The specific appearance depends on the alloy composition and the exact environmental triggers.
Common Tarnish (Oxides & Sulfides)
This is the typical discoloration seen on CNC brass parts in most indoor or industrial environments. It’s a progression that usually starts with a subtle loss of luster, moving to a light brown, then a deep chocolate brown, and eventually to a dark gray or black. This layer is primarily a mix of copper oxides, zinc oxides, and copper sulfides. While often seen as a defect in a manufacturing context, this dark, stable patina is sometimes intentionally cultivated for artistic or architectural effect. The goal in precision CNC machining is to prevent this uncontrolled formation.
Dezincification and Red Stains
In certain corrosive environments, particularly those with acidic moisture, a phenomenon called dezincification can occur. This is a selective leaching process where the more reactive zinc is pulled from the surface of the alloy, leaving behind a copper-rich layer. This can appear as reddish or pinkish stains on the surface of the brass part. This not only affects the appearance but can also weaken the material, making it porous and brittle. This is a more severe form of corrosion than simple tarnishing.
Verdigris Formation
Verdigris is the familiar greenish-blue patina seen on old bronze statues or brass fixtures exposed to the elements for many years. It is a complex mixture of hydrated copper sulfates, carbonates, and chlorides. This requires long-term exposure to moisture, carbon dioxide, and pollutants like sulfur dioxide. It is not a concern for newly manufactured CNC machined parts stored indoors but serves to illustrate the range of copper’s reactivity.
| Discoloration Type | Appearance | Primary Chemical Cause | Common Trigger |
| Tarnish | Dull, Brown to Black | Oxidation and Sulfidation | Air (Oxygen, Sulfur) |
| Dezincification | Red/Pink Stains | Selective Leaching of Zinc | Acidic Moisture, Chlorides |
| Verdigris | Green/Blue-Green | Complex Carbonates/Sulfates | Long-Term Weather Exposure |
For engineers and designers specifying CNC brass parts, preventing the initial onset of common tarnish is the primary objective.
Proactive Prevention: Steps During the CNC Machining Workflow
The most effective way to combat tarnishing is to integrate preventative measures directly into the manufacturing process at your CNC machining partner, like ly-machining.
1. Material and Alloy Selection (A DFM Approach)
From a Design for Manufacturability (DFM) perspective, choosing the right alloy is a powerful first step. Different brass alloys have different compositions and, therefore, different resistances to tarnishing.
- C360 Brass (Free-Cutting Brass): This is the most common alloy used in CNC machining due to its exceptional machinability. It has a higher lead content, which aids in chip formation. Its tarnish resistance is good for most general applications.
- C260 Brass (Cartridge Brass): This alloy has a higher copper content and no lead, making it more ductile and suitable for forming operations. However, the higher copper content can make it slightly more prone to the kind of rapid oxidation seen in pure copper.
- Naval Brass or Architectural Bronze: These specialized alloys contain additions like tin or aluminum, which significantly improve their corrosion and tarnish resistance, especially in harsh or marine environments.
Considering the end-use environment as a DFM principle can guide you to an alloy that may have a slightly higher material cost but will save significantly on post-processing or failures due to corrosion. This is a conversation to have early with your CNC milling provider.
2. Coolant Management and Immediate Cleaning
As mentioned, using a high-quality, non-staining cutting fluid formulated for yellow metals is non-negotiable. Beyond that, a strict post-machining cleaning protocol is essential. Parts should not be allowed to air dry with coolant residue on them. They should be moved immediately to a cleaning station. An ultrasonic bath with a neutral pH cleaning agent is highly effective at removing all traces of coolant, fines, and oils from the intricate features of a CNC machined part. Following the wash, a thorough drying process using filtered compressed air or a low-temperature oven is critical to remove all moisture.
3. Disciplined Handling Procedures
A clean, dry brass part is highly susceptible to contamination from human hands. The oils, salts, and moisture in a fingerprint can create a perfect environment for localized tarnishing that becomes permanently etched into the surface. All post-cleaning handling must be performed with clean, powder-free nitrile or cotton gloves. This simple process discipline is a hallmark of a quality-controlled CNC machining environment.

Protective Finishes and Coatings for Long-Term Preservation
For applications where maintaining the bright, golden appearance of brass is essential, especially for aesthetic or consumer-facing parts, applying a protective barrier is the most reliable long-term solution. This seals the surface from the environment.
1. Clear Organic Coatings (Lacquers)
The industry standard for protecting brass is a clear lacquer or acrylic clear coat. Applied via a controlled spray or dip process onto an immaculately clean part, this coating forms a thin, durable, transparent barrier. This film physically prevents oxygen, moisture, and pollutants from reaching the brass surface. The clarity of modern coatings means they are virtually invisible, preserving the natural color and luster of the CNC machined finish beneath. This is the preferred method for decorative hardware, musical instruments, and high-end electronic panels.
2. Waxes and Oils
A less permanent but still effective method is the application of a microcrystalline wax or a corrosion-inhibiting oil. The wax is buffed onto the surface, filling in the microscopic pores and creating a hydrophobic (water-repellent) layer. It imparts a warm, soft sheen and can be easily reapplied if needed. This is a good choice for parts that may need periodic maintenance. Corrosion-inhibiting oils are often used to protect parts during shipping or storage, providing a temporary shield that is easily removed with a solvent before final assembly.
3. Chemical Treatments: Passivation and Inhibitors
Passivation is a chemical treatment that encourages the formation of a thin, stable, and non-reactive surface layer. While often associated with stainless steel, chemical passivation processes exist for brass as well. These treatments can help to stabilize the surface against further oxidation. Benzotriazole (BTA) is a well-known corrosion inhibitor for copper and its alloys. It can be applied as a final rinse or dip, where it forms an invisible, chemisorbed film on the brass surface that specifically inhibits the electrochemical reactions of tarnishing. This is an excellent option for functional components where an organic coating might interfere with electrical properties or thermal transfer.
Proper Packaging: The Final Step in Prevention
A part can be perfectly machined, cleaned, and finished, but if it’s packaged improperly, it can still tarnish before it even reaches the customer. Standard packaging materials can be surprisingly detrimental.
Cardboard and non-archival papers contain acids and sulfur compounds that can leach out, causing severe contact corrosion and staining. Many standard plastic bags are permeable to moisture and can have plasticizers that react with the brass surface.
The premier solution for protecting sensitive metals is Vapor Corrosion Inhibitor (VCI) technology. VCI paper or VCI plastic bags are impregnated with a special set of chemical compounds that slowly sublimate (turn into a gas) within the sealed package. This vapor forms a protective, invisible molecular shield on the surface of the brass part. This shield blocks the corrosive effects of moisture and oxygen. When the part is unpackaged, the protective vapor layer dissipates, leaving the part clean, dry, and ready for use. For any high-value CNC machining shipment of brass parts, VCI packaging is the professional standard. Combining VCI bags with desiccant packs to absorb any trapped moisture offers the ultimate protection.
The “Aluminum CNC” Connection
While this article focuses on brass, it’s worth noting the connection to the broader world of CNC machining, often generalized by terms like “Aluminum CNC.” A CNC machine is material-agnostic; a machine that excels at cutting aluminum can also excel at cutting brass. The skills and principles are highly transferable. Both materials benefit from high-speed machining techniques. A shop, like ly-machining, that has deep expertise in machining aluminum will possess the high-speed spindles, precise controls, and robust process engineering required to machine brass effectively. The knowledge of managing thermal effects, selecting appropriate coolants, and achieving fine surface finishes learned from demanding aluminum applications is directly applicable to producing high-quality, tarnish-resistant brass components.
Related Questions
1. How do you clean a brass part that has already started to tarnish? Tarnished brass can be cleaned, but it requires care. For very light tarnish, a soft cloth with a non-abrasive metal polish can work. For more significant discoloration, commercial brass cleaners, which are typically mildly acidic, are effective. A common home remedy is a paste of lemon juice (citric acid) and salt (a mild abrasive). However, it is crucial to remember that all of these methods work by removing the tarnished layer, which also removes a minuscule amount of the base metal. On a precision CNC machined part with tight dimensional tolerances, this material removal could be problematic. Prevention is always the preferred and more professional strategy.
2. Does polishing a brass part make it more resistant to tarnishing? Polishing a brass part to a mirror finish can slow the initial rate of tarnishing, but it will not prevent it. The very smooth surface has less effective surface area and fewer sites for contaminants to lodge, giving it a temporary advantage. However, the fundamental chemical reactivity of the brass remains unchanged. A mirror-polished brass part left unprotected will still tarnish over time; it just starts from a shinier baseline. Polishing should be viewed as a surface preparation step before applying a protective coating like lacquer, not as a final protective finish in itself.
Frequently Asked Questions (FAQs)
1. Why did my brass parts turn a reddish-pink color instead of black? A reddish or pinkish discoloration on brass is a sign of dezincification. This occurs when the zinc in the alloy is selectively corroded away from the surface, leaving behind a layer that is richer in copper. This often happens in the presence of slightly acidic moisture. It can be caused by using an improper or contaminated cutting fluid during CNC milling, or by exposure to a mildly acidic environment after machining.
2. Are lead-free brass alloys more or less likely to tarnish? The lead in traditional free-cutting brass (like C360) is primarily there to improve machinability and does not play a major role in tarnish resistance. Modern lead-free brass alloys, designed to comply with regulations like RoHS, replace lead with other elements like silicon or bismuth. Their tarnish resistance is broadly comparable to traditional alloys and is primarily dictated by their copper-to-zinc ratio and the absence or presence of other protective alloying elements like tin or aluminum. The decision to use a lead-free brass is usually driven by regulatory and environmental requirements rather than tarnish prevention.
3. From a DFM perspective, when should I choose brass over aluminum? The choice between brass and aluminum is a classic Design for Manufacturability (DFM) decision. Choose brass when you need:
- Higher Density and Weight: Brass is about three times denser than aluminum, providing a feeling of substance and quality for knobs, handles, and decorative hardware.
- Specific Electrical Conductivity: Brass is highly conductive, though less so than pure copper. It’s often used for electrical contacts and connectors.
- Excellent Bearing Properties: Brass has a low coefficient of friction, making it ideal for bushings and bearings in low-load applications.
- Aesthetic Appearance: The distinct golden color of brass is often a primary design requirement. Choose aluminum when the primary drivers are light weight, high strength-to-weight ratio, and excellent corrosion resistance without the need for a coating. The experts at a CNC machining shop like ly-machining can help you weigh these trade-offs for your specific application.